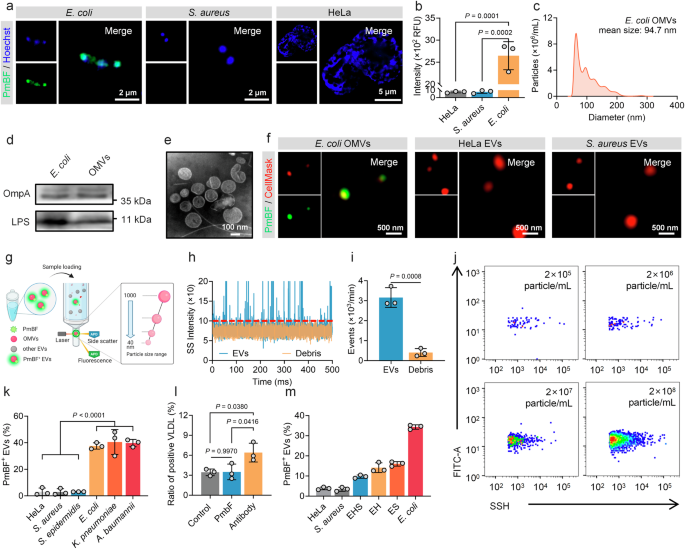
PmBF Specifically Recognizes and Quantitatively Detects OMVs
Accurate differentiation between OMVs and human EVs is a necessary prerequisite for their precise analysis. To address this challenge, we sought a molecule with high specificity for bacterial structures. Antibiotics, known for their selective targeting of microbial cells, were considered ideal options. Through preliminary literature research, Polymyxin B (PmB) emerged as a promising option due to its unique mechanism of action. This cyclic cationic polypeptide antibiotic, produced by Paenibacillus polymyxa, exerts its antibacterial effect by binding to lipopolysaccharides (LPS) on the outer membrane of Gram-negative bacteria51,52,53. We thus synthesized a fluorescent probe, PmB-FITC (PmBF), by conjugating fluorescein isothiocyanate (FITC) to the N-terminal of PmB. MALDI-TOF mass spectrometry analysis Supplementary Figs. 1a–c displayed successful coupling of PmB and FITC. Fluorescence detection and absorption spectrum of PmB before and after FITC marking similarly confirmed that the bound fluorescent probes retained their fluorescent properties Supplementary Fig. 1d. The bactericidal activity of PmBF was also evaluated, revealing a reduction in its efficacy compared to PmB Supplementary Fig. 1e. This reduction may be attributed to the structural modification introduced by FITC, which could induce conformational changes in PmB, thereby impairing its ability to insert into the bacterial membrane and exert its bactericidal function. To assess the selectivity of PmBF, we incubated it with Gram-negative bacteria (Escherichia coli, E. coli), Gram-positive bacteria (Staphylococcus aureus, S. aureus), and mammalian cells (HeLa). Super-resolution Fig. 2a and confocal images Supplementary Fig. 2 showed that PmBF signals co-localized only with E. coli, while no significant PmBF signal observed in S. aureus and HeLa. Quantitative fluorescence analysis corroborated these findings Fig. 2b, suggesting that PmBF selectively targets Gram-negative E. coli.
a Super-resolution images of Gram-negative bacteria, Gram-positive bacteria and mammal cell after incubation with PmBF for 1 h. b Fluorescence intensity of each group were detected by cytation 5, RFU: Relative Fluorescence Units (Bars represent the mean ± SD, n = 3 biological replicates). c Nanoparticles tracking analysis (NTA) of E. coli OMVs. d Western blot images of E. coli and OMVs, the protein loading amount was 50 μg for the bacterial samples and 20 μg for the EVs sample. e Transmission electron microscopy (TEM) images of E. coli OMVs. f Super-resolution images of E. coli OMVs, HeLa EVs and S. aureus EVs labeled with CellMask™ Deep Red plasma membrane stain (red) and PmBF (green). g Schematic representation of nano-flow cytometry (Created with BioRender.com). h, i Natural extracellular vesicles (control group) and debris sample detection signal and concentrations detected based on nano-flow cytometry (Bars represent the mean ± SD, n = 3 biological replicates). j Quantitative analysis of E. coli OMVs with different concentration gradients by nano-flow cytometry. k Quantitative analysis of different vesicles after PmBF labelling by nano-flow cytometry (Bars represent the mean ± SD, n = 3 biological replicates). l Quantitative analysis of positive VLDL after labeling by PmBF or antibody probes, control group was treated with PBS (Bars represent the mean ± SD, n = 3 biological replicates). m PmBF probe was used to detect E. coli OMVs within a mixture of various vesicle types (S. aureus and HeLa EVs). Group HeLa: 100% HeLa EVs; Group S. aureus: 100% S. aureus EVs; Group EHS: 40% E.coli OMVs + 30% HeLa EVs + 30% S. aureus EVs; Group EH: 50% E.coli OMVs + 50% HeLa EVs; Group ES: 50% E.coli OMVs + 50% S. aureus EVs; Group E. coli: 100% E.coli OMVs (Bars represent the mean ± SD, n = 3 biological replicates). b, k, l were determined by one-way ANOVA with multiplicity adjusted P value; i was determined by a two-tailed unpaired t-test (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns: P ≥ 0.05). Source data are provided as a Source Data file.
To confirm that PmBF achieves specific targeting through LPS, we treated E. coli with a specific antibody against LPS to competitive combined with LPS before PmBF labeling. Fluorescence imaging revealed a significant reduction in fluorescence intensity after LPS blocking Supplementary Fig. 3, indicating that PmBF’s binding to E. coli is mediated by its specific interaction with LPS. This finding further supports the specificity of PmBF for Gram-negative bacteria and suggests its potential utility in targeting OMVs, which also contain LPS.
We next investigated the ability of PmBF to target OMVs. EVs from E. coli, S. aureus and HeLa cells were isolated from the culture media by ultracentrifugation. These EVs were characterized by nanoparticle tracking analysis (NTA), western blotting (WB), and transmission electron microscopy (TEM). NTA revealed mean particle sizes of 94.7 nm, 112.4 nm, and 103.5 nm for EVs from E. coli, S. aureus, and HeLa cells, respectively Fig. 2c and Supplementary Figs. 4a, b. WB confirmed the presence of specific markers for each type of EV: OmpA and LPS for E. coli OMVs, LTA for S. aureus EVs, and TSG101 and CD81 for HeLa EVs Fig. 2d and Supplementary Figs. 4c, d, Supplementary Fig. 12. TEM imaging revealed the typical spherical structure of EVs Fig. 2e and Supplementary Figs. 4c, d. These results indicated the successful isolation of EVs by ultracentrifugation.
To evaluate PmBF’s selective binding to OMVs and visualize this interaction, EVs pre-stained with CellMask™ Deep Red plasma membrane stain were incubated with PmBF and imaged using a super-resolution microscope. PmBF signals were well colocalized with CellMask-stained OMVs from E. coli, while no significant PmBF signals were detected in the S. aureus or HeLa EV groups Fig. 2f and Supplementary Fig. 4e. These findings affirmed PmBF’s capability to specifically target E. coli-derived OMVs. Further, to accurately quantify OMVs labeled with PmBF, we introduced the nano-flow cytometry Fig. 2g, an instrument capable of analyzing nanoparticles ranging from 40 to 1000 nm. In addition, a key metric for evaluating the performance of an EV detection instrument is its ability to distinguish EVs from contaminants, such as membrane debris, which is a major source of interference and may bind to free probes, leading to false positives. To assess whether the nano-flow cytometry detection platform can exclude interference from such contaminants, we treated E. coli OMVs with Triton X-100 to generate a preparation of vesicle fragments. As shown in Fig. 2h, the signal from the debris samples was significantly lower than that of natural OMVs, with the particle number decreasing approximately 7.78 times Fig. 2i, indicating that the nano-flow cytometry can effectively exclude membrane debris for the quantitative analysis of OMVs.
Currently, OMVs are thought to be widespread in human biofluids and play key roles in various biological processes, but specific data on OMV concentrations remain limited. Most studies rely on methods like ELISA, Western blot, or Limulus amoebocyte lysate (LAL) assay, which often provide overall LPS concentrations of OMVs, not OMVs particle concentrations51,52,53,54,55,56. In our team’s previous study, we found that serum OMVs in healthy adults accounted for roughly 3% to 4% of overall EVs, approximately 3 × 107 to 4 × 1010 particles mL-157. Additionally, Gram-negative bacterial culture supernatants report OMV concentrations averaging 3.26 × 1012 particles mL-158. Here, we evaluated the sensitivity of the nano-flow cytometry platform, which was capable of detecting OMVs at concentrations as low as 2 × 105 particles mL-1 Fig. 2j, while WB detected bands only at OMV concentrations above 2 × 109 particle mL-1 Supplementary Fig. 5a. This highlighting the sensitivity and potential of nano-flow cytometry for OMV detection in these samples.
Based on this platform, We used different concentrations of PmBF probes to label the E. coli OMVs of to explore the optimal labeling concentration of PmBF, and the results are shown in Supplementary Fig. 5b. The positive rate of PmBF labeling tended to stabilize when the concentration of PmBF exceeded 5 μM. To balance labeling efficiency with minimizing non-specific binding and to maximize the signal-to-noise ratio, we selected 5 μM as the working concentration for all subsequent experiments. Following, we further expanded the types of EVs, including those derived from mammal cells, Gram-negative, and Gram-positive bacteria, to investigated the labeling efficiency of PmBF. All EVs were tested by the nano-flow cytometry after PmBF labeling. Figure 2k showed that OMVs displayed high labeling efficiency (E. coli 37.53%, K. pneumoniae 40.50%, A. baumannii 39.73%) then mammalian cells and Gram-positive bacteria-derived EVs (HeLa 3.07%, S. aureus 2.93%, S. epidermidis 3.07%). The lower labeling efficiency observed in HeLa, S. aureus, and S. epidermidis EVs might be due to nonspecific adsorption. These results further confirmed that OMVs could be specifically targeted by PmBF and quantitative analysis when combined with nano-flow cytometry.
To further evaluate PmBF’s specificity for OMVs, we investigated its ability to resist interference from lipoproteins, which are potential sources of nonspecific labeling in human samples47,48,49,50. We compared the labeling ratios of PmBF to an LPS antibody-based assay and a control group using VLDL liquid without any probes. High labeling ratios were observed in the LPS antibody group, while PmBF and the control group exhibited similar low ratios Fig. 2l. Moreover, to evaluate whether PmBF can detect OMVs in mixtures containing different types of EVs, we mixed E. coli OMVs with different types of EVs derived from HeLa or S. aureus at varying ratios (100% HeLa EVs; Group S. aureus: 100% S. aureus EVs; Group EHS: 40% E. coli OMVs + 30% HeLa EVs + 30% S. aureus EVs; Group EH: 50% E. coli OMVs + 50% HeLa EVs; Group ES: 50% E. coli OMVs + 50% S. aureus EVs; Group E. coli: 100% E. coli OMVs). The mixed samples were then analyzed to assess the ability of our method to detect and quantify OMVs. The results demonstrated that, regardless of the proportion of OMVs in the mixtures, our labeling method accurately reflected the relative proportions of OMVs Fig. 2m. Recovery rates were consistent across different mixing ratios, highlighting the sensitivity and specificity of the method in detecting OMVs in mixed EV samples. This finding demonstrates that PmBF has a higher ability to resist interference from lipoproteins and other types of EVs, highlighting its improved specificity for OMVs.
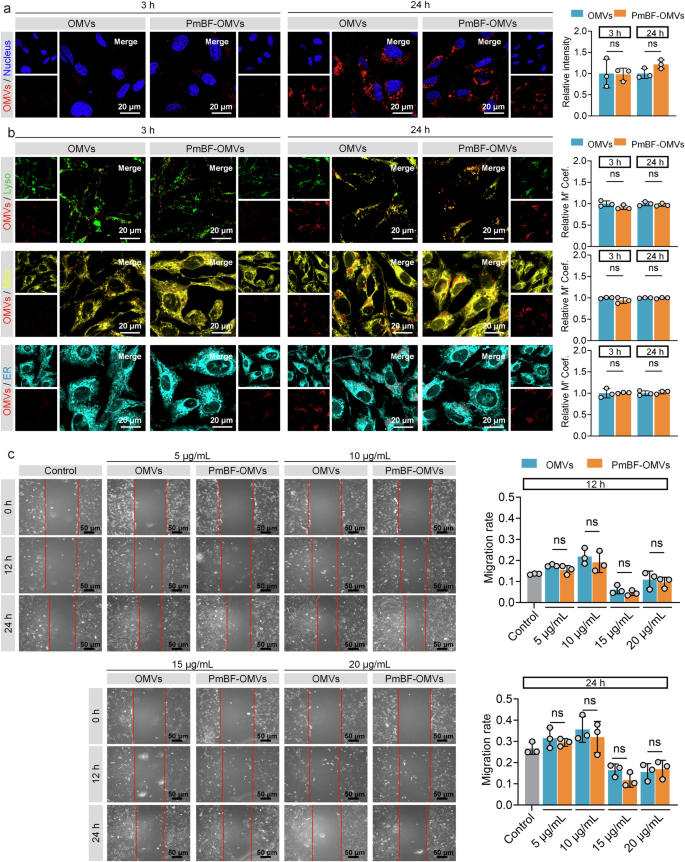
PmBF Labeling Preserves OMVs Integrity and Biological Activity
Maintaining the physical, biological, and functional integrity of OMVs during the labeling process is essential for reliable investigations of their roles in various physiological and pathological processes. To validate the suitability of PmBF for OMVs studies, we comprehensively assessed its biocompatibility, focusing on the preservation of the native properties and functions of OMVs.
First, we examined whether the labeling process alters the fundamental properties of OMVs, including their particle size, concentration, and morphology. NTA and TEM revealed that PmBF labeling preserved the size distribution and morphology of OMVs Supplementary Fig. 6a–c, presumably due to PmBF’s small molecular weight, which allows efficient labeling without causing significant physical changes to the vesicles. Additionally, we assessed the stability of PmBF-OMVs over time. The size and concentration of the PmBF-OMVs remained stable for 7 days at 4 °C, comparable to the stability of unlabeled OMVs Supplementary Fig. 6d, e. This finding demonstrated that PmBF labeling does not compromise the physical integrity of OMVs during both short-term and prolonged storage. To investigate the stability of PmBF labeling, we measured the fluorescence intensity of PmBF-OMVs over a period of 7 days Supplementary Fig. 6f. The fluorescence intensity exhibited minimal fluctuations, confirming the stable labeling capacity of PmBF probes. Furthermore, we evaluated whether PmBF labeling affects the protein composition and biological function of OMVs. Western blot analysis showed that PmBF labeling did not alter the protein composition of OMVs Supplementary Figs. 6g and 12.
Given that PmB primarily binds to the negatively charged LPS of Gram-negative bacteria59,60, we performed zeta potential measurements before and after PmBF labeling, to investigate whether the labeling concentration of the PmBF probe affects the zeta potential of OMVs. The results revealed that PmBF labeling did not significantly alter the zeta potential of OMVs Supplementary Fig. 7a. To further validate this finding, we labeled bacteria with PmBF, followed by zeta potential analysis. The results consistently showed that PmBF also didn’t cause noticeable changes in the zeta potential of the bacteria under markering concentration Supplementary Fig. 7b. To further confirm whether the observed results were affected by FITC conjugation, we conducted additional experiments using a gradient of PmB and PmBF concentrations to treat E. coli. The results showed that varying concentrations of PmB and PmBF did not significantly alter bacterial concentrations Supplementary Fig. 7c. These findings further confirmed that the probe had negligible impact on the integrity of OMVs and bacteria, indicating that PmBF labeling did not significantly interfere with the physical properties of OMVs under the experimental conditions.
For the biological activity, we assessed the OMVs by examining their effects on BEAS-2B cell proliferation. Both OMVs and PmBF-OMVs exhibited comparable effects on BEAS-2B cell proliferation at 12 h and 24 h post-coincubation Supplementary Fig. 7d. To directly observe whether PmBF labeling affects the interaction between OMVs and cells, we stained natural OMVs or PmBF-OMVs with CellMask™ Deep Red plasma membrane stain and tracked their cellular uptake. Both natural OMVs and PmBF-OMVs were successfully internalized by cells after co-incubation for 3 or 24 h, as visualized through fluorescence imaging Fig. 3a and Supplementary Fig. 7e. Quantitative fluorescence analysis further confirmed that the cellular uptake levels of natural OMVs and PmBF-OMVs were comparable, with no significant differences observed. Further, to investigate whether the intracellular distribution of OMVs is affected by PmBF labeling, different cellular organelles were stained and co-localization analysis with internalized OMVs was performed Fig. 3b and Supplementary Fig. 7f. The results showed that internalized OMVs primarily localized within lysosomes, and the distribution pattern of PmBF-OMVs was consistent with that of natural OMVs. While our current experimental setup does not allow us to definitively isolate the effect of PmbF labeling on each specific pathway, the observation that the overall internalization of OMVs is not significantly affected by PmbF labeling suggests that the major uptake mechanisms, including receptor-mediated endocytosis, are likely not substantially inhibited.
a Confocal imaging of cellular internalization of natural OMVs and PmBF-OMVs, along with quantitative analysis of fluorescence intensity, blue: nucleus; red: OMVs (Bars represent the mean ± SD, n = 3 biological replicates). b Confocal imaging of the colocalization of natural OMVs and PmBF-OMVs with different cellular organelles, accompanied by colocalization coefficients (Lyso: lysosomes, green; Mito: mitochondria, yellow; ER: endoplasmic reticulum, cyan; OMVs: red) (Bars represent the mean ± SD, n = 3 biological replicates). c Scratch experiment of BEAS-2B cells incubated with gradient concentration OMVs or labeled OMVs for 12 or 24 h, control group treated by PBS (Bars represent the mean ± SD, n = 3 biological replicates). a–c were determined by a two-tailed unpaired t-test (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns: P ≥ 0.05). Source data are provided as a Source Data file.
Additionally, to assess the potential impact of PmbF labeling on the biological activity of OMVs, we performed a wound healing scratch assay. We chose this assay based on previous studies demonstrating that OMVs can modulate cell migration and because it provides a relatively simple and quantitative readout of a complex cellular process. The results, presented in Fig. 3c, showed no significant difference in migration rates between BEAS-2B cells treated with natural OMVs and those treated with PmbF-labeled OMVs across all concentrations tested. These indicate that PmBF labeling preserves the biological activity of OMVs. Collectively, the above results highlight the stability and non-disruptive nature of PmBF labeling.
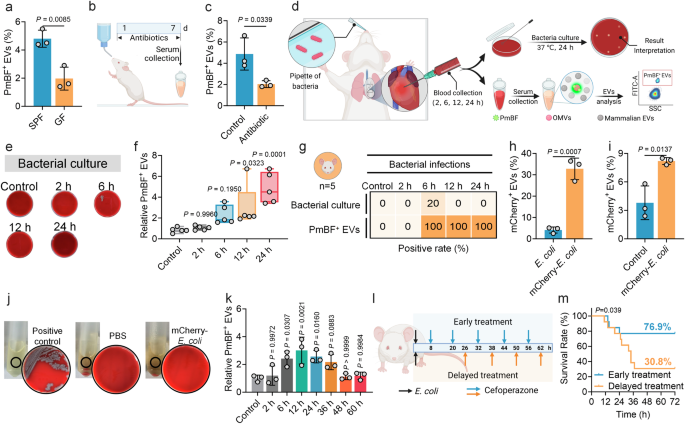
Circulating OMVs Serve as an Early Markers of Bacterial Infections
Previous studies have confirmed the presence of a small proportion of bacteria-derived OMVs in mouse and human serum57. Here, to validate whether the developed antibiotic-based fluorescent probe can detect OMVs in serum, we selected germ-free (GF, without any bacterial colonization) mice and specific pathogen-free (SPF, with normal commensal microbiota) male mice as research models. Since GF mice are theoretically devoid of bacteria, they were used as a reference to compare the detection results with those from SPF mice. The results showed that 4.80% of PmBF+ EVs on average were detected in serum from SPF mice, which was significantly higher than the 1.97% detected in GF mice Fig. 4a. To further confirm that the observed differences were caused by bacteria present in the mice, we administered a combination of antibiotics to eliminate bacteria from the mice Fig. 4b, confirmed by the remarkable reduction in fecal bacteria counting Supplementary Fig. 8a. Subsequent analysis revealed that after bacterial clearance, the proportion of positive EVs in the treated mice was significantly reduced to 2.03% Fig. 4c, near the levels of GF mice. These results demonstrate that our developed method can effectively identify OMVs in serum and suggest a correlation between the presence of OMVs in mouse serum and the bacterial load within the body.
a Analysis of circulating PmBF+ EVs levels in germ-free (GF) and conventional mice (SPF) mice by nano-flow cytometry (Bars represent the mean ± SD, n = 3 biological replicates). b Schematic diagram of the construction of a mouse model of colonized bacteria clearance, d: days (Created with BioRender.com). c Analysis of circulating PmBF+ EVs levels in mice models before (Control group) or after intestinal flora cleared (Bars represent the mean ± SD, n = 3 biological replicates). d Schematic diagram of the construction of mouse models of bacterial infections and blood were collected from mice after intranasally injection with 8 × 107 CFU bacteria at 2, 6, 12, 24 h (Created with BioRender.com). e Blood bacterial culture plates for mouse models after E. coli infections for 2, 6, 12, 24 h, control group mice was treated with PBS. f Quantitative analysis of circulating PmBF+ EVs changes in mouse models after E. coli infections for 2, 6, 12, 24 h, control group mice was treated with PBS (The center line of each box indicates the median. The bottom and top bonds of the box show the 25th and 75th percentiles, respectively. Whiskers extend to the minimum and maximum values, n = 5 biological replicates). g Comparison of bacterial cultures and circulating OMVs positivity rates in mouse models after E. coli infections for 2, 6, 12, 24 h, control group mice was treated with PBS (Elements Created with BioRender.com). h Expression analysis of mCherry in wide-type or mCherry-E. coli derived OMVs (Bars represent the mean ± SD, n = 3 biological replicates). i Analysis of circulating mCherry+ EVs in mice models infected with mCherry-E. coli, control group mice was treated with PBS. The Y-axis indicates the percentage of serum mCherry+ EVs to total EVs. (Bars represent the mean ± SD, n = 3 biological replicates). j Blood bacterial culture plates as well as LB medium. Positive control: live mCherry-E. coli; PBS: blood of mice treated by PBS; mCherry-E. coli: blood of mice treated by mCherry-E. coli. No viable bacterial colonies were observed in group PBS and mCherry-E. coli. k Dynamic monitoring of circulating PmBF+ EVs levels at different time points post-infection in mice. Antibiotic treatment was initiated at 12 h post-infection, with administration every 12 h thereafter (Bars represent the mean ± SD, n = 3 biological replicates). l Illustration of the effect of different points in time of treatment on mouse infection models survival rate (Created with BioRender.com). m Mouse survival curves. a, c, h, i was determined by a two-tailed unpaired t-test; f, k were determined by one-way ANOVA with multiplicity adjusted P value; Survival analysis was performed using the Log-rank (Mantel-Cox) test to compare the survival curves between groups (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns: P ≥ 0.05). Source data are provided as a Source Data file.
We further tested this correlation in bacterial infections. We developed a male mouse model of lung infection by intranasally introducing two most common pathogens, E. coli or K. pnenmoniae Fig. 4d. Blood samples were collected at different time points post-infection for bacterial culture and serum EVs detection. In the E. coli infection group, serum PmBF+ EVs levels significantly increased at 6, 12 and 24 h post-infection Figs. 4e, f, while only one mouse had a positive bacterial culture at 6 h. The positive rates of serum PmBF+ EVs were consistently higher than those of bacterial cultures Fig. 4g. Commonly used inflammatory markers, CRP and PCT, showed variable elevations Supplementary Figs. 8b, c, with PCT not significantly increasing at 6, 12, and 24 h and CRP not significantly increasing at 12 h. Similarly, in the K. pneumoniae infection group, PmBF+ EVs were showed elevated trends at 6, 12 and 24 h post-infection, with all negative blood bacterial cultures Supplementary Figs. 8d–f. To further assess the specificity of PmBF for detecting Gram-negative bacterial infections, we developed a mouse infection model using the Gram-positive bacterium Streptococcus pneumoniae (S. pneumoniae). Circulating EVs were analyzed 12 h post-infection. As shown in Supplementary Fig. 8g, the levels of PmBF+ EVs in the serum of S. pneumoniae-infected mice remained unchanged. These results provide additional evidence supporting the specificity of PmBF for the diagnosis of Gram-negative bacterial infections.
Additionally, to investigate the bacterial load required to affect circulating OMVs levels, we infected mice with varying bacterial concentrations and measured circulating OMVs. The results revealed that at a bacterial load of 8 × 106 CFU, circulating OMVs levels in some mice began to plateau. At bacterial loads down to 8 × 105 CFU, circulating OMVs levels in all mice were comparable to those of uninfected controls Supplementary Fig. 8h.
To determine whether these elevated circulating PmBF+ EVs originated directly from the infecting bacteria at the site of infection, rather than from increased intestinal OMV release due to potential gut barrier dysfunction, we engineered an E. coli strain expressing an mCherry-OmpA fusion protein. Red fluorescence intensity was significantly elevated in mCherry-E. coli compared to wild type E. coli Supplementary Fig. 9, indicating the successful plasmid introduction. Further validation revealed that OMVs from mCherry-E. coli carried mCherry, as evidenced by a higher fluorescent rate Fig. 4h. In the lung infection model, mice intranasally administered with mCherry-E. coli exhibited an average of 8.2% mCherry+ vesicles in serum at 6 h post-infection, significantly higher than in PBS-treated mice Fig. 4i. Similarly, no viable mCherry-E. coli were cultured from the blood of infected mice Fig. 4j. Taken together, these findings demonstrate that the elevated circulating PmBF+ OMVs mainly originated from the infecting bacteria and highlight the potential of PmBF+ EVs as sensitive markers for bacterial infections.
Further, to investigate the dynamic changes in circulating OMVs levels during bacterial infection and their association with infection resolution, we infected mice with bacteria and monitored circulating OMVs levels over time. Antibiotic treatment was initiated at 12 h post-infection to assess the impact of infection remission on OMVs dynamics. As shown in Fig. 4k, circulating OMVs levels began to rise at 6 h post-infection and peaked at 12 h, indicating an active bacterial infection. Upon initiation of antibiotic treatment, circulating OMVs levels gradually decreased over time. As the treatment progresses, OMVs levels declined gradually compared to the peak levels, and by 36-48 h, they returned to baseline levels comparable to those in uninfected controls. These results suggest that circulating OMVs levels dynamically reflect the progression of bacterial infection and respond to effective antibiotic treatment, highlighting their potential as biomarkers for monitoring infection and treatment efficacy.
To investigate the impact of early diagnosis on therapeutic outcomes, we constructed mouse lung infection models and initiated antibiotic treatment at different time points, with dosing every 12 h, and observed the survival rates Fig. 4l. In the delayed treatment group, the survival rate dropped to 30.8% at 32 h, while it was effectively increased to 76.9% in the early treatment group Fig. 4m. These findings demonstrate the importance of early intervention in improving prognosis. Notably, in these mouse lung infection models, PmBF+ EVs were detectable in the circulation as early as 6 h post-infection, preceding positive bacterial cultures and significant elevations in conventional inflammatory markers. This early detection of PmBF+ EVs highlights their potential to facilitate timely diagnosis and guide early treatment decisions, which can significantly improve patient outcomes.
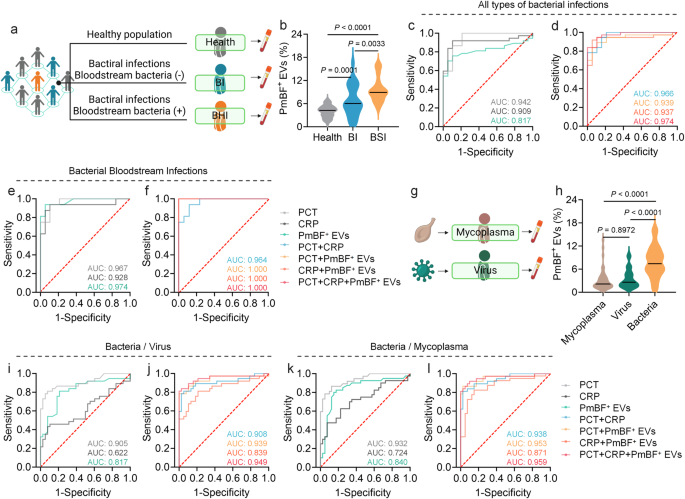
Clinical Utility of PmBF+ EVs as Biomarkers for Bacterial Infections
To explore the clinical potential of PmBF+ EVs as a biomarker, we analyzed serum samples from healthy individuals and patients with bacterial infections, both with negative (BI) and positive (BSI) bloodstream cultures Fig. 5a and Supplementary Table 1. Serum EVs were isolated and characterized by NTA, WB, and TEM Supplementary Figs. 10 and 12. As showed in Fig. 5b, the PmBF+ EVs levels in patient serum were significantly elevated compared to healthy controls (mean 7.90% vs. 4.00%), with BSI patients exhibiting higher levels (mean 9.61%, range 5.00-15.90%) than BI patients (mean 6.75%, range 0.70-16.50%), suggesting a correlation between PmBF+ EV levels and infection severity.
a Clinical samples collection of healthy people and patients with different types of bacterial infections (Created with BioRender.com). b Detection of circulating PmBF+ EVs levels in the health screening population and patients with different types of bacterial infections (The horizontal line indicates the median, Health: n = 60 biological replicates; BI: n = 24 biological replicates; BSI: n = 16 biological replicates). c, d ROC curves for the diagnosis of all types of infections by PmBF+ EVs, CRP, PCT alone and in combination. e, f ROC curves for the diagnosis of bacterial bloodstream infections by PmBF+ EVs, CRP, PCT alone and in combination. g Clinical samples collection of patients with mycoplasma or virus infections (Created with BioRender.com). h Detection of circulating PmBF+ EVs levels in patients with mycoplasma, virus or bacterial infections (include bacterial infections with positive or negative blood cultures) (The horizontal line indicates the median, Mycoplasma: n = 40 biological replicates; Virus: n = 38 biological replicates; Bacteria: n = 40 biological replicates). i, j ROC curve for differential diagnosis between bacterial or virus infections. k, l ROC curve for differential diagnosis between bacterial or mycoplasma infections. b, h were determined by one-way ANOVA with multiplicity adjusted P value (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns: P ≥ 0.05). Source data are provided as a Source Data file.
To further investigate the diagnostic performance of PmBF+ EVs, receiver operating characteristic (ROC) curve analyses were conducted. For all bacterial infections and bloodstream infections specifically, the areas under the curve (AUC) were 0.817 and 0.974, respectively Fig. 5c, e. Notably, combining PmBF+ EVs with PCT and CRP further improved the AUC for bacterial infection diagnosis Fig. 5d, particularly for bacterial bloodstream infections Fig. 5f.
Finally, we investigated the potential of PmBF+ EVs in differentiating bacterial infections from viral and mycoplasma infections Fig. 5g and Supplementary Table 1. Circulating PmBF+ EV levels were significantly higher in patients with bacterial infections compared to those with virus or mycoplasma infections Fig. 5h. ROC analyses for differential diagnosis yielded AUCs of 0.817 (bacteria vs. virus) and 0.840 (bacteria vs. mycoplasma) for PmBF+ EVs alone Fig. 5i, k. Combining PmBF+ EVs with PCT and CRP further improved the AUCs to 0.949 and 0.959, respectively, highlighting the potential of PmBF+ EVs as a biomarker for the differential diagnosis of bacterial infections.