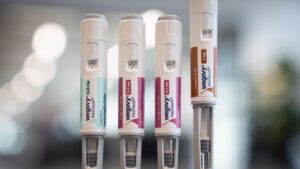
The World Well being Group (WHO) has granted Emergency Use Listing (EUL) for the LC16m8 mpox vaccine, making it the second mpox vaccine to be supported by WHO following the Director-Basic’s declaration of an mpox public well being emergency of worldwide concern (PHEIC) on 14 August 2024.
This resolution is predicted to facilitate elevated and well timed entry to vaccines in communities the place mpox outbreaks are surging. In 2024, instances have been reported throughout 80 international locations, together with 19 international locations in Africa, based on data as of 31 October 2024. The Democratic Republic of the Congo, the hardest-hit nation, recorded a big majority of suspected instances – over 39 000 – in addition to greater than 1000 deaths.
At the moment’s transfer is especially related because the Authorities of Japan has introduced that it’s going to donate 3.05 million doses of the LC16m8 vaccine, together with specialised inoculation needles, to the Democratic Republic of the Congo. That is the biggest donation package deal introduced so far in response to the present mpox emergency.
LC16m8 is a vaccine developed and manufactured by KM Biologics in Japan. The Technical Advisory Group (TAG) for EUL of vaccines convened to debate the end result of the LC16m8 vaccine evaluation, together with the product and programmatic suitability assessments. The TAG recommended the vaccine to be used in people over one yr of age as a single dose vaccine, by way of a a number of puncture approach utilizing a bifurcated needle.
“WHO emergency use itemizing of the LC16m8 vaccine towards mpox marks a major step in our response to the present emergency, offering a brand new choice to guard all populations, together with youngsters,” mentioned Dr Yukiko Nakatani, WHO Assistant Director-Basic for Entry to Medicines and Well being Merchandise. “Vaccines are one of many essential instruments to assist include the outbreak as a part of a complete response technique that additionally consists of improved testing and analysis, therapy and care, an infection prevention management, and engagement and schooling inside affected communities.”
WHO’s evaluation for EUL is predicated on data submitted by the producer and evaluation by the Prescription drugs and Medical Gadgets Company (PMDA), the Japanese regulatory company of document for this vaccine. The LC16m8 vaccine has been utilized in Japan throughout earlier mpox outbreaks and was proven to be protected and efficient, together with in folks with well-controlled HIV.
The WHO Strategic Advisory Group of Consultants (SAGE) on Immunization reviewed accessible proof and recommended the usage of LC16m8 vaccine in outbreak settings in youngsters and others with a documented high-risk of publicity to mpox.
Nonetheless, minimally replicating vaccines, reminiscent of LC16m8, shouldn’t be used throughout being pregnant and in people who find themselves immunocompromised. Immunocompromised individuals embrace these with energetic most cancers, transplant recipients, immunodeficiency, and energetic therapy with immunosuppressive brokers. In addition they embrace folks residing with HIV with a present CD4 cell rely of <200 cells µl.
The World Advisory Committee on Vaccine Security reviewed the up to date security knowledge on LC16m8 on 20 September 2024 and beneficial that healthcare staff are supplied with coaching on the use of bifurcated needles to stop accidents and antagonistic results. In gentle of the altering epidemiology and emergence of recent virus strains, it stays essential to gather as a lot knowledge as attainable on vaccine security and effectiveness in several contexts.
WHO continues to work carefully with producers, world companions and international locations to make sure the supply and administration of protected and efficient life-saving merchandise.
On 13 September 2024, WHO prequalified the Modified Vaccinia Ankara-Bavarian Nordic (MVA-BN) vaccine and expanded its indication to incorporate use in people aged 12 years and older on 8 October 2024.
Be aware to editors:
WHO Prequalification (PQ) and Emergency Use Itemizing (EUL) are mechanisms used to guage high quality, security and efficacy of medical merchandise, reminiscent of vaccines, diagnostics and medicines, and product suitability to be used within the contexts of low- and middle-income international locations. Merchandise receiving PQ or EUL help decision-making for worldwide, regional and nation procurement by UN and accomplice procurement businesses and Member States. PQ is predicated on the evaluation of full set of high quality, security and efficacy knowledge on medical merchandise, together with danger administration plan and programmatic suitability. EUL is a danger profit evaluation to handle pressing calls for throughout public well being emergencies primarily based on accessible restricted knowledge the place the advantages outweigh the dangers.
![[original_title]](https://rawnews.com/wp-content/uploads/2024/11/mpox-drc-2024.tmb-1200v-1024x681.jpg)