The World Well being Group (WHO) has introduced the MVA-BN vaccine as the primary vaccine in opposition to mpox to be added to its prequalification record.
The prequalification approval is predicted to facilitate well timed and elevated entry to this important product in communities with pressing want, to scale back transmission and assist comprise the outbreak. WHO’s evaluation for prequalification is predicated on info submitted by the producer, Bavarian Nordic A/S, and evaluation by the European Medicines Company, the regulatory company of report for this vaccine.
“This primary prequalification of a vaccine in opposition to mpox is a vital step in our combat in opposition to the illness, each within the context of the present outbreaks in Africa, and in future,” mentioned WHO Director-Common Dr Tedros Adhanom Ghebreyesus. “We now want pressing scale up in procurement, donations and rollout to make sure equitable entry to vaccines the place they’re wanted most, alongside different public well being instruments, to forestall infections, cease transmission and save lives.”
The MVA-BN vaccine could be administered in folks over 18-years of age as a 2-dose injection given 4 weeks aside. After prior chilly storage, the vaccine could be stored at 2–8°C for as much as 8 weeks.
“The WHO prequalification of the MVA-BN vaccine will assist speed up ongoing procurement of the mpox vaccines by governments and worldwide companies equivalent to Gavi and Unicef to assist communities on the frontlines of the continuing emergency in Africa and past,” mentioned Dr Yukiko Nakatani, WHO Assistant Director-Common for Entry to Medicines and Well being Merchandise. “The choice may assist nationwide regulatory authorities to fast-track approvals, finally growing entry to quality-assured mpox vaccine merchandise.”
The WHO Strategic Advisory Group of Specialists (SAGE) on Immunization reviewed all out there proof and recommended using MVA-BN vaccine within the context of an mpox outbreak for individuals at excessive danger of publicity. Whereas MVA-BN is at present not licensed for individuals beneath 18 years of age, this vaccine could also be used “off-label” in infants, kids and adolescents, and in pregnant and immunocompromised folks. This implies vaccine use is really useful in outbreak settings the place the advantages of vaccination outweigh the potential dangers.
WHO additionally recommends single-dose use in supply-constrained outbreak conditions. WHO emphasizes the necessity to acquire additional information on vaccine security and effectiveness in these circumstances.
Out there information reveals {that a} single-dose MVA-BN vaccine given earlier than publicity has an estimated 76% effectiveness in defending folks in opposition to mpox, with the 2-dose schedule attaining an estimated 82% effectiveness. Vaccination after publicity is much less efficient than pre-exposure vaccination.
Good security profile and vaccine efficiency has been constantly demonstrated in medical research, in addition to in real-world use through the ongoing world outbreak since 2022. In gentle of the altering epidemiology and emergence of latest virus strains, it stays essential to gather as a lot information as potential on vaccine security and effectiveness in several contexts.
Because the triggering of the emergency use itemizing for mpox vaccines by WHO Director-Common on 7 August 2024, WHO has carried out product and programmatic suitability assessments of MVA-BN vaccine.
“The findings of the assessments are notably related within the context of the declaration of a public well being emergency of worldwide concern (PHEIC) associated to the upsurge of mpox in Africa,” mentioned Dr Rogerio Gaspar, WHO Director for Regulation and Prequalification. “We’re progressing with prequalification and emergency use itemizing procedures with producers of two different mpox vaccines: LC-16 and ACAM2000. We now have additionally acquired 6 expressions of curiosity for mpox diagnostic merchandise for emergency use itemizing to this point.”
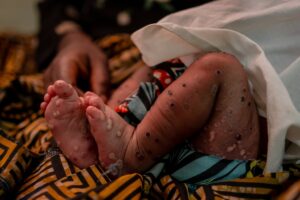
The escalating mpox outbreak within the Democratic Republic of the Congo and different international locations was declared a PHEIC by the WHO Director-Common on 14 August 2024.
Over 120 international locations have confirmed greater than 103 000 instances of mpox for the reason that onset of the worldwide outbreak in 2022. In 2024 alone, there have been 25 237 suspected and confirmed instances and 723 deaths from completely different outbreaks in 14 international locations of the African Area (primarily based on information from 8 September 2024).
Editor’s observe:
WHO Prequalification (PQ) and Emergency Use Itemizing (EUL) are mechanisms used to judge high quality, security and efficacy of medical merchandise, equivalent to vaccines, diagnostics and medicines (optionally available: together with biotherapeutics), and product suitability to be used in an low- and middle-income nation context. PQ or EUL listed merchandise help determination for worldwide, regional and nation procurement by UN and associate procurement companies and member states. PQ is predicated on the evaluation of full set of high quality, security and efficacy information on medical merchandise, together with danger administration plan and programmatic suitability. EUL is a danger profit evaluation to handle pressing calls for throughout public well being emergencies primarily based on out there restricted information. Underneath EUL, the producers are required to decide to proceed producing lacking info to fulfil prequalification necessities. As soon as this info turns into out there, a PQ utility ought to be submitted to finish the complete course of to realize suggestion for worldwide procurement in each emergency and non-emergency settings.
![[original_title]](https://rawnews.com/wp-content/uploads/2024/09/drc-mpox-vaccine-arrival.tmb-1200v-1024x576.jpg)