Roche raised hopes this yr that it had a future blockbuster drug on its arms after early trial outcomes of the Swiss pharmaceutical group’s new weight problems therapies confirmed fast weight reduction amongst recipients.
However revelations earlier this week of excessive charges of vomiting and different negative effects amongst those that took sturdy doses of the medicine have unsettled traders and highlighted the challenges going through companies that wish to enter the profitable new marketplace for “GLP-1” medicine.
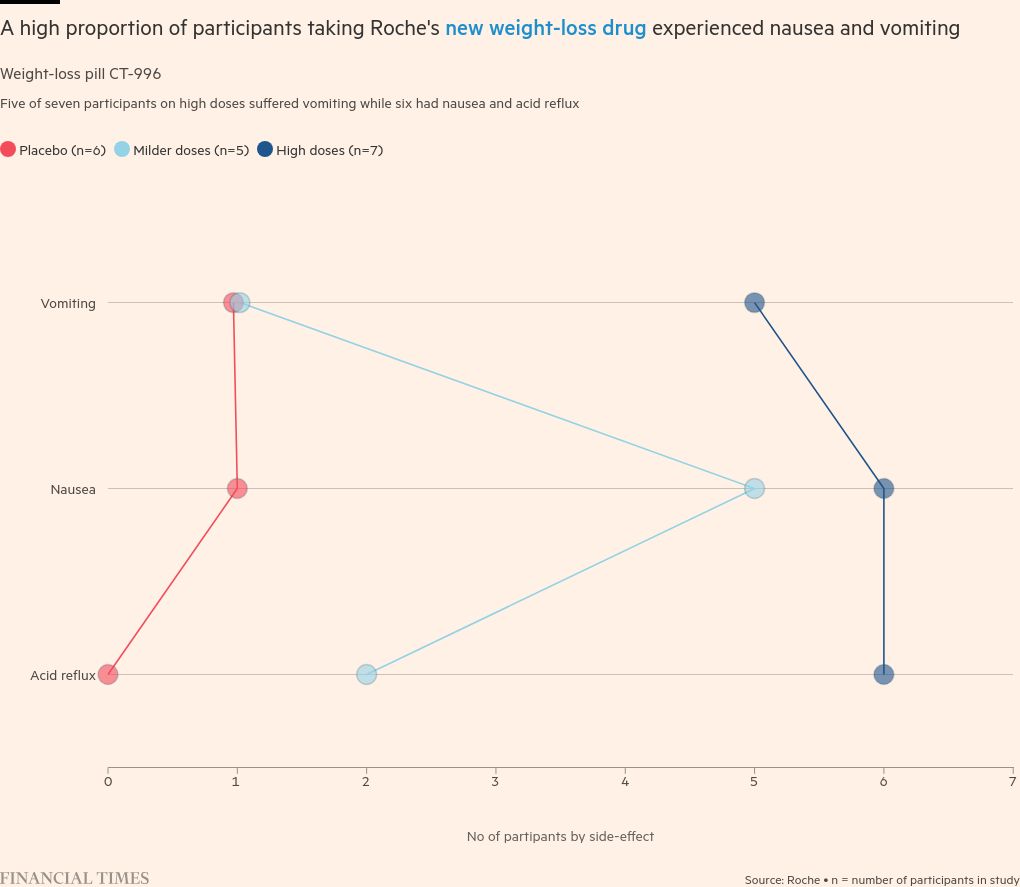
The corporate’s shares dropped 4 per cent on Monday after it revealed that three-quarters of sufferers on the very best dose of its CT-388 injection had suffered from vomiting. They fell one other 5 per cent on Thursday after related information for its oral weight-loss capsule.
The response is a reminder that not all sufferers can tolerate the brand new class of weight-loss therapies dominated by Novo Nordisk and Eli Lilly — and that challengers to the trade’s pioneers face vital hurdles.
The therapies have been one of many principal speaking factors on the convention of the European Affiliation for the Examine of Diabetes in Madrid this week.
Peter Verdult, a Citigroup analyst attending the convention, stated Roche had brought on issues for itself by touting its preliminary trial leads to July as “actually particular information”.
“I don’t suppose anybody can say that now,” he stated. “They set themselves up for a fall.”
International drugmakers are racing to meet up with Novo Nordisk and Eli Lilly’s lead within the profitable and quickly rising GLP-1 drug market.
Of the 1,150 analysis abstracts introduced on the Madrid convention, virtually one-Tenth featured GLP-1 medicine.
The treatment has proved an efficient approach each to regulate weight and deal with diabetes, and Goldman Sachs analysts have estimated the marketplace for the merchandise may develop to $130bn yearly by 2030.
The novel medicine work by mimicking the intestine hormone GLP-1, which lowers blood sugar and limits the urge for food. Remedies equivalent to Eli Lilly’s Mounjaro additionally add one other intestine hormone, GIP, that seems to boost weight reduction. As well as, firms are experimenting with different intestine and pancreatic hormones.
Francine Kaufman, a former head of the American Diabetes Affiliation and now chief medical officer of medical units firm Senseonics, stated the medicine had revolutionised diabetes care at a second when weight problems charges have been rising.
“I stated within the 2000s that we wanted a silver bullet,” she stated. “It arrived.”
GLP-1-based medicine are, however, related to vomiting, nausea and constipation, notably in increased doses. They’re additionally linked with muscle wastage in some trials. The US Meals and Drug Administration and the European Medicines Company have explored different extra severe side-effects reported with latest medicine — equivalent to suicidal ideas — however discovered no proof of a hyperlink.
Defending the potential of Roche’s medicine, Manu Chakravarthy, who leads the corporate’s metabolic product improvement, stated it had needed to “push the tolerability” of its drug and that side-effects at excessive doses have been per different GLP-1-based medicine.
Customers of Roche’s merchandise in future trials have been unlikely to obtain such excessive doses or fast will increase, Chakravarthy added.
“We’re inspired because it can’t get any worse than this,” he stated.
Drugmakers use early trials to check the protection of their medicine and sometimes take a look at excessive doses. Verdult stated there have been additionally issues about side-effects years in the past when Novo Nordisk and Eli Lilly introduced information on their medicine.
On the Madrid convention, Novo Nordisk introduced information on a brand new drug — oral amycretin — displaying it prompted vomiting in additional than half of customers on the very best dose.
The side-effects seem to discourage some sufferers. Analysis printed this yr by Blue Well being Intelligence discovered that 30 per cent of GLP-1 customers stopped remedy inside 4 weeks of beginning, with negative effects a big issue. Price and availability of the medicine are additionally an element.
Former UK prime minister Boris Johnson wrote in a Every day Mail newspaper column final yr that he was unable to tolerate vomiting linked to taking diabetes remedy Ozempic off-label.
Different drugmakers have had setbacks in creating so-called small molecule tablets — artificial medicine which can be simpler to fabricate at scale than weight-loss injections.
Pfizer deserted a twice-daily model of its weight-loss drug danuglipron after recording a excessive diploma of nausea and vomiting in mid-stage trials. However the New York-based drugmaker is pushing forward with a day by day, tweaked model.
To deal with negative effects, firms are creating various formulations of medication. Analysts famous pleasure about amylin, a pancreatic hormone that’s thought to scale back muscle wastage linked to medicine, though this has but to be proved at scale.
Novo Nordisk’s subsequent product — CagriSema — combines GLP-1s with an amylin analogue. The drugmaker will report late-stage information later this yr from the product.
Eli Lilly has struck a number of offers aimed toward resolving the difficulty of muscle wastage. Final yr it spent as much as $1.9bn buying Versanis, whose lead drug is predicated on the hormone activin that helps to manage muscle mass. It is usually partnering with BioAge, an organization creating a muscle regeneration drug. BioAge just lately filed for an preliminary public providing.
But the rising information continues to underline how a lot firms, traders and scientists nonetheless have to find about how GLP-1 therapies work.
Among the many research introduced in Madrid was one which confirmed Eli Lilly’s Mounjaro drug led to more practical weight reduction amongst ladies than males but additionally brought on increased charges of nausea and vomiting.
Luis-Emilio García-Perez, the Eli Lilly scientist who undertook that examine, stated the corporate didn’t know why the outcomes differed between the 2 teams.
Ilya Yuffa, Eli Lilly’s head of worldwide operations, stated it was nonetheless unclear whether or not new therapies would have fewer negative effects and would enable the anticipated broad take-up of the medicine.
“For the opposite molecules which can be in improvement which will have new approaches, I believe it’s in all probability too early to have a transparent view of what they seem like in broader populations,” stated Yuffa.